CDMO (contract research / manufacturing)

In the future, we plan to move our R&D and manufacturing base from our current Amagasaki factory to our new Moriyama factory. We will strengthen our system so that we can meet a wider range of customer needs.
We contribute to realizing our customers' requests with our formulation technology, polymer synthesis technology, and production technology.
Formulation technology
We design by introducing various technologies related to formulation, such as optimizing the type and amount of additives and arranging the state of drug dissolution and dispersion.
Polymer synthesis technology
We have multiple proprietary pharmaceutical pressure sensitive adhesives, which are key materials for transdermal patches, and propose high-performance formulations. In addition, it is also possible to take advantage of our strengths as a general-purpose pressure sensitive adhesives manufacturer * 1 to synthesize new products. * 2
* 1. Generic pressure sensitive adhesives Product Page
* 2.If a new pressure sensitive adhesives is developed, it falls under the category of a new additive and must be approved as part of the formulation
Industrial science
We comply with PIC/S GMP and propose optimal manufacturing plans based on our many years of manufacturing experience. It can be manufactured from clinical trial level to commercial production level.
Image of role sharing with customers (during joint development)
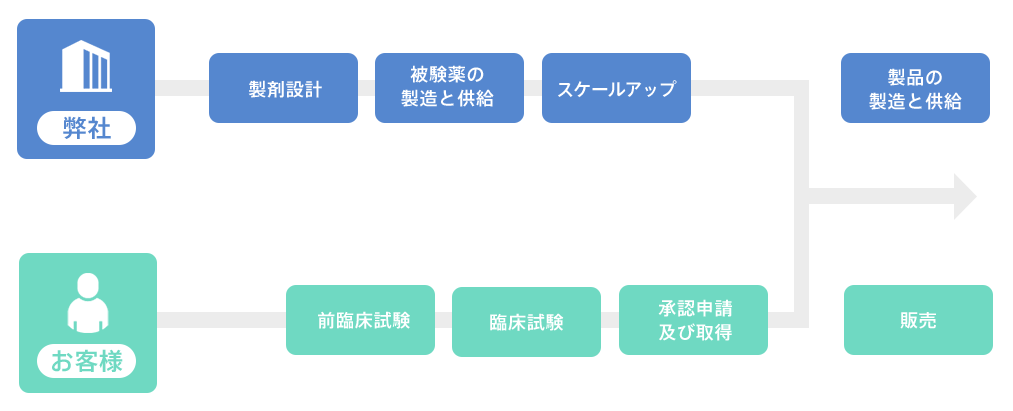
Contract research and development
We will propose a plan that meets your needs, such as feasibility studies for transdermal patches and consideration of investigational formulations. In addition to medical drugs, we also accept consultations regarding quasi-drugs and cosmetics.
Contract research flow

Example of work

Transdermal absorbability
Evaluate whether the drug of interest has transdermal absorption.
Skin irritation
Assess whether there is any skin irritation caused by the drug itself.

Additive selection and trial production
We make full use of our unique polymer technology and compounding technology to design and prototype samples according to customer requests.
Evaluation of formulation performance
We evaluate whether drug requirements are met, such as drug release, stability, adhesion, and absorption.

Sample manufacturing
We can manufacture and supply investigational drugs under GMP from various evaluation samples.
Contract manufacturing
TOYOCHEM responds to a wide range of customer needs, from planning to manufacturing of transdermal patches. transdermal patches can be manufactured in an integrated manner, from mixing, coating, slitting, punching, filling, and packaging.
The supply amount will vary greatly depending on the customer's product specifications, so we will decide upon consultation with you.
Contract manufacturing process

Manufacturing process

Inquiries
TOYOCHEM CO., LTD. Medical Science Unit
