What is transdermal absorption technology?
- Classification of transdermal patches
- Mechanism of transdermal absorption
- Precautions for formulation
- Current status of transdermal patches
Classification of transdermal patches
transdermal patches are classified as external preparations, and there are two types: tapes and poultices.
| Tape agent | transdermal patches that uses a base that contains almost no water. |
|---|---|
| Poultice | transdermal patches that uses a base containing water. |
Furthermore, by focusing on the structure, the following classifications are possible.
| Matrix type | It is transdermal patches with a relatively simple structure: a plaster layer containing an even mixture of drugs, pressure sensitive adhesives, and additives is applied to a support, and a liner layer is placed on top to protect the plaster layer. There are also products with separate drug and pressure sensitive adhesives layers. |
|---|---|
| Reservoir type | It is transdermal patches with a structure that includes a release control layer sandwiched between a drug storage layer and an pressure sensitive adhesives layer. Control layer provides stable drug release. |
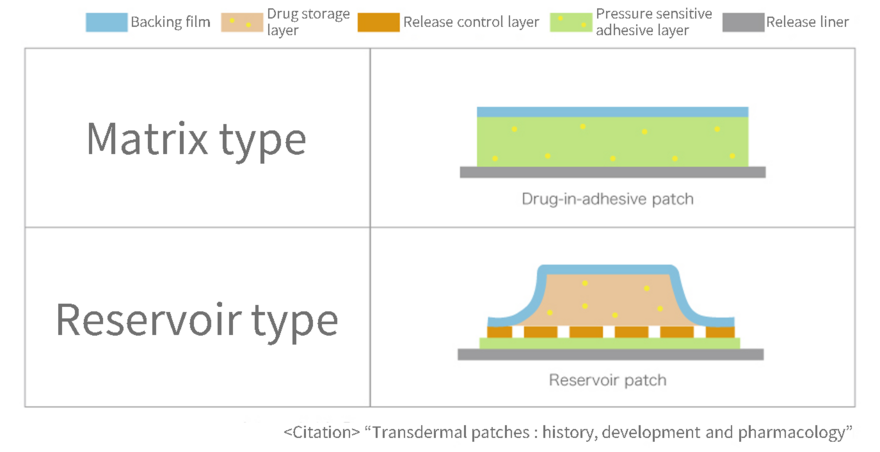
As shown in the figure, matrix-type formulations have a simple structure, so they have the advantage of being relatively easy to manufacture and reducing the thickness of the formulation. Most of transdermal patches currently being developed are matrix-type.
The following classifications based on the scope of action are also possible.
| Locally acting type | This includes anti-inflammatory analgesics that are applied to the affected area to suppress inflammation and pain. The medicinal effect is limited to the area where it is pasted. |
|---|---|
| Systemically acting type | This is transdermal patches that releases the drug into the blood through the skin and acts throughout the body through blood circulation. |
Mechanism of transdermal absorption
Transdermal drug absorption route
The diagram on the right shows the transdermal absorption route for drugs. It is thought that it passes not only through relatively large pores such as sweat glands and pores, but also through the interior of corneocytes and intercellular lipids.
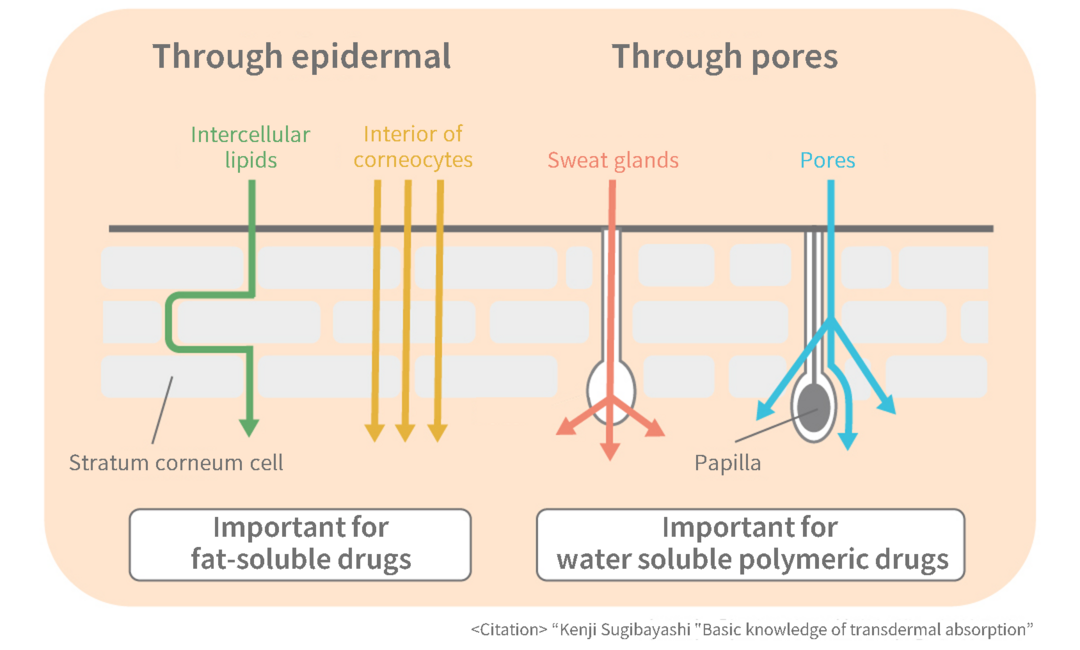
In order to pass through this route, drugs that can be formulated into transdermal patches meet the following conditions:
| Molecular weight | Drugs that can penetrate the stratum corneum, which has a barrier function, have a limited molecular weight. Many of the drugs that have currently been successfully formulated into transdermal patches have a molecular weight of 500 or less. |
|---|---|
| Moderate fat solubility | Cell walls are mainly composed of fat, so it is known that substances with moderate fat solubility are easily absorbed by cells. Focusing on the oil-water partition coefficient of drugs that have been made transdermal patches, it is said that logP is approximately between 1 and 4.5. |
| Melting point | When absorbed into the skin, drugs are absorbed as molecules. Substances with low melting points have more active molecules and are therefore more easily absorbed into the body. |
Precautions for formulation
transdermal patches an pressure sensitive adhesives layer that is applied directly to the skin to absorb the active ingredients, so safety for the human body is extremely important.
Skin irritation
If the sheet does not adhere well to the skin or if there is residual solvent, it may cause symptoms such as skin irritation or itching. Also, caution is required as the drug itself may be irritating to the skin.
Easy to apply and remove
With transdermal patches, you can go about your daily life while wearing them. It is important that it does not come off with a little exercise, and that it can be removed without pain when you want to remove it. Also, if the adhesive is too strong, there is a risk of peeling off the stratum corneum when peeling it off.
Current status of transdermal patches
The table below shows (excerpt) systemically acting transdermal patches currently on the market.
(0: released, x: not released)
| Disease | Common name | Japan | Europe and America | Product shape | Molecular weight | Melting point ℃ |
|---|---|---|---|---|---|---|
| Bronchial asthma | Tulobuterol | 〇 | × | Matrix type | 227.7 | 90~93 |
| Ischemic heart disease | Nitroglycerin | 〇 | 〇 | Reservoir type Matrix type |
227.1 | 13.5 |
| Isosorbide nitrate | 〇 | × | Matrix type | 236.1 | 70 | |
| High blood pressure | Clonidine | × | 〇 | Reservoir type | 230.1 | 130 |
| bisoprolol | 〇 | × | Matrix type | 325.4 | 29 | |
| Overactive bladder | Oxybutynin hydrochloride | 〇 | 〇 | Matrix type | 393.9 | 129~130 |
| Alzheimer's disease | Rivastigmine | 〇 | 〇 | Matrix type | 250.3 | - |
| Parkinson's disease | Rotigotine | 〇 | 〇 | Matrix type | 315.5 | 94~100 |
| Attention Deficit/Hyperactivity Disorder | Methylphenidate | × | 〇 | Matrix type | 269.8 | 204 |
| Schizophrenia | Blonanserin | 〇 | × | Matrix type | 367.5 | 123~126 |
| Depression | Selegiline | × | 〇 | Matrix type | 223.7 | 140~144 |
| Postmenopausal osteoporosis | estradiol | 〇 | 〇 | Reservoir type | 272.4 | 173~179 |
| Nausea | scopolamine | × | 〇 | Reservoir type | 303.4 | 59 |
| Allergic rhinitis | emedastine fumarate | 〇 | × | Reservoir type | 534.6 | 149~152 |
| Pain relief | fentanyl | 〇 | 〇 | Reservoir type Matrix type |
336.5 | 83~84 |
| Pain relief No smoking |
Buprenorphine | 〇 | 〇 | Matrix type | 348.9 | 291 |
| nicotine | 〇 | 〇 | Reservoir type | 162.2 | 90 |
Although there are fewer types of transdermal patches compared to other dosage forms, formulations are progressing in various fields. The simplicity of medication administration and ease of management is a major benefit for patients, so further development is expected in the future.
References
“Basics and practical application in the development of transdermal formulations” Supervised by: Kenji Sugibayashi, Josai University
Request when making an inquiry
If you belong to a company, research institute, or other corporation, please contact us with the name of the corporation and the URL of the website.
* Please note that we do not respond to inquiries from individuals.
Inquiries
TOYOCHEM CO., LTD. Medical Science Unit
